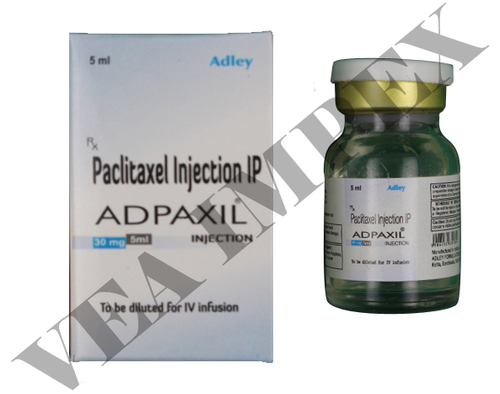


Adpaxil 30 mg(Paclitaxel Injection)
Product Details:
- Origin of Medicine India
- Salt Composition Paclitaxel
- Drug Type Injection
- Physical Form Liquid
- Function Anti-Cancer
- Dosage 30 mg
- Storage Instructions Cool and Dry place
- Click to view more
Adpaxil 30 mg(Paclitaxel Injection) Price And Quantity
- 5000-1000 Piece
- 1085 INR/Piece
Adpaxil 30 mg(Paclitaxel Injection) Product Specifications
- Cool and Dry place
- Anti-Cancer
- Injection
- India
- Liquid
- 30 mg
- Paclitaxel
Adpaxil 30 mg(Paclitaxel Injection) Trade Information
- 10000 Piece Per Week
- 7-15 Days
- South America Middle East Africa Central America Eastern Europe Western Europe Asia North America Australia
- All India
Product Description
Product name Adpaxil 30 mg
Generic Name Paclitaxel Injection
Manufacturer Adley
Adpaxil belongs to the group of medicinescalled antineoplastics It interferes with the growth of cancer cells whichare eventually destroyed Since the growth of normal body cells may also beaffected other unwanted effects will also occur Some of these may be seriousand must be reported to your doctor Other effects may not be serious but maycause concern Some effects may not occur until months or years after themedicine is used
HOWSHOULD I USE ADPAXIL
Use Adpaxil as directed by your doctor Check the label on themedicine for exact dosing instructions
USESOF ADPAXIL IN DETAILS
Adpaxil is used alone or in combination withother chemotherapeutic agents in the treatment of ovarian cancer breastcancer advanced nonsmall cell lung cancer cancer of pancreas and AIDSrelated Kaposis sarcoma a type of cancer that develops from the cells thatline lymph or blood vessels
ADPAXILINTERACTIONS
Ketoconazole As ketoconazole may inhibit the metabolism of Adpaxilpatients receiving Adpaxil and ketoconazole should be closely monitored or thecombination of these drugs should be avoided
ADPAXILSIDE EFFECTS
Hematologic Bonemarrow suppression was the major doselimiting toxicity of AdpaxilNeutropenia the most important hematologic toxicity was dose andscheduledependent and was generally rapidly reversible Among patients treatedin phase 3 secondline ovarian study with a 3hr infusion neutrophil countsdeclined 500 cellsmm3 in 14 of patients treated with adose of 135 mgm2compared to 27 at a dose of 175 mgm2 p005

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+

 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese





 Call Me Free
Call Me Free
